Review Article | Vol. 9 Issue 4 (2026), e2026151 | Published in 09 September 2025
Applications of inoculants to treat drought stress in plants
Link: https://10.31893/multirev.2026151
- AbstractAll climatic factors, including temperature, rainfall, humidity, wind, and solar radiation, significantly influence agricultural activities. These environmental conditions determine the types of crops that can be grown, the length of growing seasons, and the overall productivity of agricultural systems. While some agricultural areas around the world rely on artificial irrigation, the vast majority still depend heavily on natural rainfall patterns. This reliance makes agriculture particularly vulnerable to climate variability and change. Climate change is expected to increase the frequency and severity of extreme weather events, such as droughts and heatwaves, which will lead to higher water demands from crops while simultaneously reducing water availability. This imbalance between water supply and demand is likely to result in reduced crop yields and lower production capacity in many regions. One of the most critical challenges faced by agriculture under climate change is drought. Drought stress severely limits plant growth and productivity by affecting physiological and biochemical processes. However, plant growth-promoting bacteria (PGPB) offer a promising solution to this issue. These beneficial microbes enhance plant tolerance to drought by regulating gene expression and modulating hormone activity in plants. They also influence the stress-induced enzymatic system, alter phytohormone levels, and contribute to the accumulation of protective metabolites. These mechanisms are expressed through phenotypic changes in plant architecture, growth rate, root-to-shoot ratio, hydraulic conductivity, and water conservation abilities. Additionally, PGPB support plant cell protection under stressful conditions. This review aims to highlight the role of plant growth-promoting bacteria in mitigating the adverse effects of climate-induced stress on crops. By exploring how these microorganisms interact with plants to enhance resilience, it provides insight into potential applications of microbial biotechnology in agroecosystems. Ultimately, the integration of such microbial strategies into farming practices can contribute to more sustainable and climate-resilient agriculture.
Keywords:
sustainable agriculture
biofertilizers
PGPB
endophytic bacteria
abiotic stress
inoculant
- References
- Abbasi, S., Sadeghi, A., & Safaie, N. (2020). Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Scientia Horticulturae, 265, 109206. https://doi.org/10.1016/j.scienta.2020.109206
- Aguiar, N. O., Medici, L. O., Olivares, F. L., Dobbss, L. B., Torres-Netto, A., Silva, S. F., Novotny, E. H., & Canellas, L. P. (2016). Metabolic profile and antioxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria. Annals of Applied Biology, 168, 203–213. https://doi.org/10.1111/aab.12256
- Ahmed, A., & Hasnain, S. (2010). Auxin-producing Bacillus sp.: Auxin quantification and effect on the growth of Solanum tuberosum. Pure and Applied Chemistry, 82, 313–319. https://doi.org/10.1351/PAC-CON-09-02-06
- Ahmed, B., Shahid, M., Syed, A., Rajput, V. D., Elgorban, A. M., Minkina, T., Bahkali, A. H., & Lee, J. (2021). Drought tolerant Enterobacter sp./Leclercia adecarboxylata secretes indole-3-acetic acid and other biomolecules and enhances the biological attributes of Vigna radiata (L.) R. Wilczek in water deficit conditions. Biology, 10, 1149. https://doi.org/10.3390/biology10111149
- Akhtar, M. S., & Siddiqui, Z. A. (2009). Effect of phosphate solubilizing microorganisms and Rizobium sp. on the growth, nodulation, yield and root-rot disease complex of chickpea under field condition. African Journal of Biotechnology, 8, 3489–3496. http://www.academicjournals.org/AJB
- Akram, N. A., Waseem, M., Ameen, R., & Ashraf, M. (2016). Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: Some key physio-biochemical traits. Acta Physiologiae Plantarum, 38, 3. https://doi.org/10.1007/s11738-015-2018-1
- Alam, M. M., Nahar, K., Hasanuzzaman, M., & Fujita, M. (2014). Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics, 7, 271–283. https://www.pomics.com/hassanuzzaman_7_4_2014_271_283.pdf
- Alam, S., Khalil, S., Ayub, N., & Rashid, M. (2022). In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganism (PSM) from maize rhizosphere. International Journal of Agriculture and Biology, 4, 454–458. http://www.ijab.org
- Ali, B., Wang, X., Saleem, M. H., Sumaira Hafeez, A., Afridi, M. S., Khan, S., Zaib Un, N., Ullah, I., & Amaral Júnior, A. T. D. (2022). PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants, 11, 345. https://doi.org/10.3390/plants11030345
- Arzanesh, M. H., Alikhani, H. A., Khavazi, K., Rahimian, H. A., & Miransari, M. (2011). Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World Journal of Microbiology and Biotechnology, 27, 197–205. https://doi.org/10.1007/s11274-010-0444-1
- Ashraf, M. (2010). Inducing drought tolerance in plants: Recent advances. Biotechnology Advances, 28, 169–183. https://doi.org/10.1016/j.biotechadv.2009.11.005
- Baldotto, L. E. B., Baldotto, M. A., Canellas, L. P., Bressan-Smith, R., & Olivares, F. L. (2010). Growth promotion of pineapple ‘Vitória’ by humic acids and Burkholderia spp. during acclimatization. Revista Brasileira de Ciência do Solo, 34, 1593–1600. https://doi.org/10.1590/S0100-06832010000500012
- Bandurska, H. (2022). Drought stress responses: Coping strategy and resistance. Plants, 11, 922. https://doi.org/10.3390/plants11070922
- Bashan, Y., Holguin, G., & De-Bashan, L. (2004). Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances. Canadian Journal of Microbiology, 50, 521–577. https://doi.org/10.1139/w04-035
- Becker, D., Hoth, S., Ache, P., Wenkel, S., Roelfsema, M. R., Meyerhoff, O., Hartung, W., & Hedrich, R. (2003). Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Letters, 554(1–2), 119–126. https://doi.org/10.1016/S0014-5793(03)01118-9
- Berg, G. (2009). Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Applied Microbiology and Biotechnology, 84, 11–18. https://doi.org/10.1007/s00253-009-2092-7
- Bhat, M. I., Rashid, A., Faisul-Ur, R., Mahdi, S. S., Haq, S. A., & Raies, A. B. (2010). Effect of Rhizobium and vesicular-arbuscular mycorrhizae fungi on green gram (Vigna radiata L. Wilczek) under temperate conditions. Research Journal of Agricultural Sciences, 1, 113–118. https://www.scirp.org/reference/referencespapers?referenceid=669377
- Bisleski, R. L. (1973). Phosphate transport and phosphate availability. Annual Review of Plant Physiology, 24, 225–252. https://doi.org/10.1146/annurev.pp.24.060173.001301
- Bittencourt, P. P., Alves, A. F., Ferreira, M. B., da Silva Irineu, L. E. S., Pinto, V. B., & Olivares, F. L. (2023). Mechanisms and applications of bacterial inoculants in plant drought stress tolerance. Microorganisms, 11(2), 502. https://doi.org/10.3390/microorganisms11020502
- Blom, D., Fabbri, C., Connor, E. C., Schiestl, F. P., Klauser, D. R., Boller, T., Eberl, L., & Weisskopf, L. (2011). Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environmental Microbiology, 13, 3047–3058. https://doi.org/10.1111/j.1462-2920.2011.02582.x
- Brilli, F., Loreto, F., & Baccelli, I. (2019). Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Frontiers in Plant Science, 10, 264. https://doi.org/10.3389/fpls.2019.00264
- Broeckling, C. D. (2008). Root exudates regulate soil fungal community composition and diversity. Applied Environmental Microbiology, 74, 738–744. https://doi.org/10.1128/AEM.02188-07
- Brundrett, M. C., & Abbott, L. K. (1995). Mycorrhizal fungus propagules in the jarrah forest. I. Spatial variability in inoculum levels. New Phytologist, 131, 461–469. https://doi.org/10.1111/j.1469-8137.1995.tb03083.x
- Champawat, R. S., & Pathak, V. N. (1993). Effect of vesicular-arbuscular mycorrhizal fungi on growth and nutrition uptake of pearl millet. Indian Journal of Mycology and Plant Pathology, 23, 30–34. https://doi.org/10.1104/pp.102.3.771
- Chang, B., Yang, L., Cong, W., & Tang, Z. (2014). The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiology and Biochemistry, 77, 140–148. https://doi.org/10.1016/j.plaphy.2014.02.001
- Chen, H., & Jiang, J. G. (2010). Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environmental Reviews, 18, 309–319. https://doi.org/10.1139/A10-014
- Cho, S. M., Kang, B. R., Han, S. H., Anderson, A. J., Park, J. Y., Lee, Y. H., Cho, B. H., Yang, K. Y., Ryu, C. M., & Kim, Y. C. (2008). 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Molecular Plant-Microbe Interactions, 21, 1067–1075. https://doi.org/10.1094/MPMI-21-8-1067
- Cochard, H., Coll, L., Le Roux, X., & Améglio, T. (2002). Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiology, 128, 282–290. https://pmc.ncbi.nlm.nih.gov/articles/PMC148995/
- Cohen, A. C., Bottini, R., & Piccoli, P. N. (2008). Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regulation, 54, 97–103. https://doi.org/10.1007/s10725-007-9232-9
- Constable, G. A., & Hearn, A. B. (1978). Agronomic and physiological responses of soybean and sorghum crops to water deficits I. Growth, development and yield. Functional Plant Biology, 5, 159–167. https://doi.org/10.1071/PP9780159
- De Souza, R., Schoenfeld, R., & Passaglia, L. M. P. (2016). Bacterial inoculants for rice: Effects on nutrient uptake and growth promotion. Archives of Agronomy and Soil Science, 62, 561–569. https://doi.org/10.1080/03650340.2015.1065973
- Deubel, A., Gransee, G., & Merbach, W. (2000). Transformation of organic rhizodeposits by rhizoplane bacteria and its influence on the availability of tertiary calcium phosphate. Journal of Plant Nutrition and Soil Science, 163, 387–392. https://doi.org/10.1002/1522-2624(200008)163:4
- Duchense, L. C., Peterson, R. L., & Ellis, B. E. (1989). The future of ectomycorrhizal fungi as biological control agents. Phytoprotection, 70, 51–57. https://doi.org/10.1007/978-3-642-85063-9_3
- Dumas, G. E., Guillaume, P., Tahiri, A. A., Gianinazzi-Pearson, V., & Gianinazzi, S. (1994). Changes in polypeptide patterns in tobacco roots by Glomus species. Mycorrhiza, 4, 215–221. https://link.springer.com/book/10.1007/978-94-017-0776-3
- Duponnois, R., Kisa, M., & Plenchette, C. (2006). Phosphate solubilizing potential of the nemato fungus Arthrobotrys oligospora. Journal of Plant Nutrition and Soil Science, 169, 280–282. https://doi.org/10.1002/jpln.200520551
- Dutton, V. M., & Evans, C. S. (1996). Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Canadian Journal of Microbiology, 42, 881–895. https://doi.org/10.1139/m96-114
- El-Komy, H. M., Hamdia, M. A., & El-Baki, G. K. A. (2003). Nitrate reductase in wheat plants grown under water stress and inoculated with Azospirillum spp. Biologia Plantarum, 46, 281–287. https://doi.org/10.1023/A:1022819114860
- El-Komy, M., & Hesham, A. (2004). Coimmobilization of Azospirillum lipoferum and Bacillus megaterium for successful phosphorus and nitrogen nutrition of wheat plants. Food Technology and Biotechnology, 43, 19–27. https://www.ftb.com.hr/images/pdfarticles/2005/January-March/43-19.pdf
- Etesami, H., & Maheshwari, D. K. (2018). Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicology Environment Safety, 156, 225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
- Fa Yuan, W., & Zhao Yong, S. (2008). Biodiversity of arbuscular mycorrhizal fungi in China: A review. Advances in Environmental Biology, 2, 31–39. https://www.aensiweb.com/old/aeb/2008/31-39.pdf
- Fierer, N., & Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences of the USA, 103, 626–631. https://doi.org/10.1073/pnas.0507535103
- Foley, J. A., Ramankutty, N., Brauman, K. A., Cassidy, E. S., Gerber, J. S., Johnston, M., Mueller, N. D., O’Connell, C., Ray, D. K., & West, P. C. (2011). Solutions for a cultivated planet. Nature, 478, 337–342. https://doi.org/10.1038/nature10452
- Fulchieri, M., & Frioni, L. (1994). Azospirillum inoculation on maize (Zea mays): Effect on yield in a field experiment in Central Argentina. Soil Biology and Biochemistry, 26, 921–924. https://doi.org/10.1016/0038-0717(94)90308-5
- Garg, S., Bhatnagar, A., Kalla, A., & Narula, N. (2001). In vitro nitrogen fixation, phosphate solubilization, survival and nutrient release by Azotobacter strains in an aquatic system. Bioresource Technology, 80, 101–109. https://doi.org/10.1016/s0960-8524(01)00081-5
- Geurts, R., & Bisseling, T. (2002). Rhizobium nod factor perception and signalling. The Plant Cell, 14, S239–S249. https://doi.org/10.1105/tpc.002451
- Ghosh, D., Gupta, A., & Mohapatra, S. (2019). A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World Journal of Microbiology and Biotechnology, 35(90). https://doi.org/10.1007/s11274-019-2659-0
- Glick, B. R., Penrose, D. M., & Li, J. (1998). A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. Journal of Theoretical Biology, 190, 63–68. https://doi.org/10.1006/jtbi.1997.0532
- Greacen, E. L., & Oh, J. S. (1972). Physics of root growth. Nat. New Biol., 235, 24–25. https://doi.org/10.1038/newbio235024a0
- Gull, M., Hafeez, F. E., Saleem, M., & Malik, K. A. (2004). Phosphorus uptake and growth promotion of chickpea by co-inoculation of mineral phosphate solubilizing bacteria and a mixed rhizobial culture. Australian Journal of Experimental Agriculture, 44, 623–628. https://doi.org/10.1071/EA02218
- Gullino, P., Luca, B., & Federica, L. (2018). Linking multifunctionality and sustainability for valuing peri-urban farming: A case study in the Turin Metropolitan Area (Italy). Sustainability, 10(5), 1625. https://doi.org/10.3390/su10051625
- Habibi, A., Heidari, G., Sohrabi, Y., Badakhshan, H., & Mohammadi, K. (2011). Influence of bio, organic and chemical fertilizers on medicinal pumpkin traits. Journal of Medicinal Plants Research, 5, 5590–5597. http://www.academicjournals.org/JMPR
- Harb, A., Awad, D., & Samarah, N. (2015). Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. Journal of Plant Interactions, 10, 109–116. https://doi.org/10.1080/17429145.2015.1033023
- Hart, M. M., & Reader, R. J. (2002). Host plant benefit from association with arbuscular mycorrhizal fungi: Variation due to differences in size of mycelium. Biology and Fertility of Soils, 36, 357–366. https://doi.org/10.1007/s00374-002-0539-4
- Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline under changing environments: A review. Plant Signaling & Behavior, 7, 1456–1466. https://doi.org/10.4161/psb.21949
- Henri, F., Laurette, N. N., Annette, A., John, Q., Wolfgang, M., François-Xavier, E., & Dieudonné, E. (2008). Solubilization of inorganic phosphates and plant growth promotion by strains of Pseudomonas fluorescens isolated from acidic soils of Cameroon. African Journal of Microbiology Research, 2, 171–178. https://www.internationalscholarsjournals.com/articles/solubilization-of-inorganic-phosphates-and-plant-growth-promotion-by-strains-of-pseudomonas-fluorescens-isolated-from-ac.pdf
- Hernández, I., Cela, J., Alegre, L., & Munné-Bosch, S. (2012). Antioxidant defenses against drought stress. In R. Aroca (Ed.), Plant responses to drought stress: From morphological to molecular features (pp. 231–258). Springer. https://doi.org/10.1007/978-3-642-32653-0_9
- Hilda, R., & Fraga, R. (2000). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances, 17, 319–359. https://doi.org/10.1016/s0734-9750(99)00014-2
- Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant and Soil, 237, 173–195. https://doi.org/10.1023/A:1013351617532
- Hochberg, U., Windt, C. W., Ponomarenko, A., Zhang, Y. J., Gersony, J., Rockwell, F. E., & Holbrook, N. M. (2017). Stomatal closure, basal leaf embolism, and shedding protect the hydraulic integrity of grape stems. Plant Physiology, 174, 764–775. https://doi.org/10.1104/pp.16.01816
- Honma, M., & Shimomura, T. (1978). Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agricultural and Biological Chemistry, 42, 1825–1831. https://doi.org/10.1080/00021369.1978.10863261
- Hungria, M., Campo, R. J., Souza, E. M., & Pedrosa, F. O. (2010). Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant and Soil, 331, 413–425. https://doi.org/10.1007/s11104-009-0262-0
- Hussein, H. A. A., Mekki, B. B., Abd El-Sadek, M. E., & El Lateef, E. E. (2019). Effect of L-ornithine application on improving drought tolerance in sugar beet plants. Heliyon, 5, e02631. https://doi.org/10.1016/j.heliyon.2019.e02631
- Igual, J. M., Valverde, A., Cervantes, E., & Velázquez, E. (2001). Phosphate-solubilizing bacteria as inoculants for agriculture: Use of updated molecular techniques in their study. Agronomie, 21, 561–568. https://hal.science/hal-00886151/document
- Iqbal, S., Wang, X., Mubeen, I., Kamran, M., Kanwal, I., Díaz, G. A., Abbas, A., Parveen, A., Atiq, M. N., & Alshaya, H. (2022). Phytohormones trigger drought tolerance in crop plants: Outlook and future perspectives. Frontiers in Plant Science, 12, 3378. https://doi.org/10.3389/fpls.2021.799318
- Jeffries, A. (1987). Use of mycorrhiza in agriculture. Critical Reviews in Biotechnology, 5, 319–357. https://doi.org/10.3109/07388558709079476
- Jjemba, P. K., & Alexander, M. (1999). Possible determinants of rhizosphere competence of bacteria. Soil Biology and Biochemistry, 31, 623–632. https://doi.org/10.1016/S0038-0717(98)00168-0
- Jochum, M. D., McWilliams, K. L., Borrego, E. J., Kolomiets, M. V., Niu, G., Pierson, E. A., & Jo, Y. K. (2019). Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Frontiers in Microbiology, 10, 2106. https://doi.org/10.3389/fmicb.2019.02106
- Junaid, M. D., Gokce, A. F., & Bostani, R. (2024). Global agricultural losses and their causes. Bulletin of Biological and Allied Science Research, 9(1), 66. https://doi.org/10.54112/bbasr.v2024i1.66
- Kang, S. M., Shahzad, R., Bilal, S., Khan, A. L., Park, Y. G., Lee, K. E., Asaf, S., Khan, M. A., & Lee, I. J. (2019). Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiology, 19, 80. https://doi.org/10.1186/s12866-019-1450-6
- Kasim, W. A., Osman, M. E. H., Omar, M. N., & Salama, S. (2021). Enhancement of drought tolerance in Triticum aestivum L. seedlings using Azospirillum brasilense NO40 and Stenotrophomonas maltophilia B11. Bulletin of the National Research Centre, 45, 95. https://doi.org/10.1186/s42269-021-00546-6
- Kathiresan, G., Manickam, G., & Parameswaran, P. (1995). Efficiency of phosphobacteria addition on cane yield and quality. Cooperative Sugar, 26, 629–631. https://www.academia.edu/77310716/Bacterial_biofertilizers_for_sustainable_crop_production_a_review
- Kavamura, V. N., Santos, S. N., Silva, J. L. D., Parma, M. M., Ávila, L. A., Visconti, A., Zucchi, T. D., Taketani, R. G., Andreote, F. D., & Melo, I. S. D. (2013). Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiological Research, 168, 183–191. https://doi.org/10.1016/j.micres.2012.12.002
- Khan, A. L., Halo, B. A., Elyassi, A., Ali, S., Al-Hosni, K., Hussain, J., Al-Harrasi, A., & Lee, I. J. (2016). Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electronic Journal of Biotechnology, 21, 58–64. http://dx.doi.org/10.1016/j.ejbt.2016.02.001
- Khan, I. A., Ayub, N., Mirza, S. N., Nizami, S. M., & Azam, M. (2008). Synergistic effect of dual inoculation (vesicular-arbuscular mycorrhizae) on the growth and nutrient uptake of Medicago sativa. Pakistan Journal of Botany, 40, 939–945. https://www.researchgate.net/publication/235913809_Synergistic_Effect_of_Dual_Inoculation_Vesicular_Arbuscular_Mycorrhizae_on_the_Growth_and_Nutrients_Uptake_of_Medicago_sativa
- Khan, M. S., Zaidi, A., & Wani, P. A. (2007). Role of phosphate-solubilizing microorganisms in sustainable agriculture: A review. Agronomy for Sustainable Development, 27, 29–43. https://doi.org/10.1051/agro:2006011
- Khan, N., & Bano, A. (2019). Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE, 14, e0222302. https://doi.org/10.1371/journal.pone.0222302
- Khosro, M., & Yousef, S. (2012). Bacterial biofertilizers for sustainable crop production: A review. ARPN Journal of Agricultural and Biological Science, 7, 307–316. https://www.arpnjournals.com/jabs/research_papers/rp_2012/jabs_0512_396.pdf
- Kim, K. Y., Jordan, D., & McDonald, G. A. (1998). Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biology and Fertility of Soils, 26, 79–87. https://doi.org/10.1007/s003740050347
- Kpomblekou, K., & Tabatabai, M. A. (1994). Effect of organic acids on release of phosphorus from phosphate rocks. Soil Science, 158, 442–453. https://journals.lww.com/soilsci/abstract/1994/15860/effect_of_organic_acids_on_release_of_phosphorus.6.aspx
- Kumar, A., & Verma, J. P. (2018). Does plant—microbe interaction confer stress tolerance in plants: A review? Microbiological Research, 207, 41–52. https://doi.org/10.1016/j.micres.2017.11.004
- Lee, K. E., & Pankhurst, C. E. (1992). Soil organisms and sustainable productivity. Australian Journal of Soil Research, 30, 855–892. http://dx.doi.org/10.1071/SR9920855
- Li, C., Li, L., Reynolds, M. P., Wang, J., Chang, X., Mao, X., & Jing, R. (2021). Recognizing the hidden half in wheat: Root system attributes associated with drought tolerance. Journal of Experimental Botany, 72, 5117–5133. http://dx.doi.org/10.1093/jxb/erab124
- Malhi, G. S., Kaur, M., & Kaushik, P. (2021). Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability, 13, 1318. https://doi.org/10.3390/su13031318
- McAfee, B. J., & Fortin, J. A. (1986). Comparative effects of the soil microflora on ectomycorrhizal inoculation of conifer seedling. New Phytologist, 108, 108–443. https://doi.org/10.1111/j.1469-8137.1988.tb04185.x
- Mohammadi Alagoz, S., Zahra, N., Hajiaghaei Kamrani, M., Asgari Lajayer, B., Nobaharan, K., Astatkie, T., Siddique, K. H. M., & Farooq, M. (2022). Role of root hydraulics in plant drought tolerance. Journal of Plant Growth Regulation, 1–16. https://research-repository.uwa.edu.au/en/publications/role-of-root-hydraulics-in-plant-drought-tolerance
- Moreno-Galván, A. E., Cortés-Patiño, S., Romero-Perdomo, F., Uribe-Vélez, D., Bashan, Y., & Bonilla, R. R. (2020). Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Applied Soil Ecology, 147, 103367. https://doi.org/10.1016/j.apsoil.2019.103367
- Nahas, E. (1996). Factors determining rock phosphate solubilization by microorganisms isolated from soil. World Journal of Microbiology and Biotechnology, 12, 18–23. https://doi.org/10.1007/BF00327716
- Narayanasamy, S., Thankappan, S., Kumaravel, S., Ragupathi, S., & Uthandi, S. (2023). Complete genome sequence analysis of a plant growth-promoting phylloplane Bacillus altitudinis FD48 offers mechanistic insights into priming drought stress tolerance in rice. Genomics, 115, 110550. https://doi.org/10.1016/j.ygeno.2022.110550
- Naveed, M., Mitter, B., Reichenauer, T. G., Wieczorek, K., & Sessitsch, A. (2014). Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environmental Experimental Botany, 97, 30–39. https://doi.org/10.1016/j.envexpbot.2013.09.014
- Nessner Kavamura, V., Santos, S. N., da Silva, J. L., Parma, M. M., Aparecida Ávila, L., Visconti, A., Zucchi, T. D., Gouvêa Taketani, R., Andreote, F. D., & de Melo, I. S. (2013). Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiological Research, 168(3), 183–191. https://doi.org/10.1016/j.micres.2012.12.002
- Nihorimbere, V., Ongena, M., Smargiassi, M., & Thonart, P. (2011). Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnologie Agronomie Société et Environnement, 15, 327–337. https://popups.uliege.be/1780-4507/index.php?id=7578
- Nivetha, N., Lavanya, A. K., Vikram, K. V., Asha, A. D., Sruthi, K. S., Bandeppa, S., Annapurna, K., & Paul, S. (2021). PGPR-mediated regulation of antioxidants: Prospects for abiotic stress management in plants. In Plant Stress Physiology: From Genomics to Systems Biology (pp. 471–497). Springer. https://doi.org/10.1007/978-981-16-1350-0_23
- Ojuederie, O. B., Olanrewaju, O. S., & Babalola, O. O. (2019). Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy, 9, 712. https://doi.org/10.3390/agronomy9110712
- Olivares, F. L., Busato, J. G., De Paula, A. M., Da Silva Lima, L., Aguiar, N. O., & Canellas, L. P. (2017). Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chemical and Biological Technologies in Agriculture, 4, 30. https://doi.org/10.1186/s40538-017-0112-x
- Pantoja-Guerra, M., Valero-Valero, N., & Ramírez, C. A. (2023). Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: A review. Chemical and Biological Technologies in Agriculture, 10, 6. https://doi.org/10.1186/s40538-022-00370-8
- Patil, P. L., & Medhane, N. S. (1994). Seed inoculation studies in gram (Cicer arietinum L.) with different strains of Rhizobium sp. Plant and Soil, 40, 221–223. https://doi.org/10.1007/bf00011425
- Ponmurugan, P., & Gopi, G. (2006). Distribution pattern and screening of phosphate solubilizing bacteria isolated from different food and forage crops. Journal of Agronomy, 5, 600–604. https://doi.org/10.3923/ja.2006.600.604
- Poudel, M., Mendes, R., Costa, L. A. S., Bueno, C. G., Meng, Y., Folimonova, S. Y., Garrett, K. A., & Martins, S. J. (2021). The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Frontiers in Microbiology, 12, 3058. https://doi.org/10.3389/fmicb.2021.743512
- Pyngrope, S., Bhoomika, K., & Dubey, R. S. (2013). Oxidative stress, protein carbonylation, proteolysis and antioxidative defense system as a model for depicting water deficit tolerance in Indica rice seedlings. Plant Growth Regulation, 69, 149–165. https://doi.org/10.1007/s10725-012-9758-3
- Rao, D. E., & Chaitanya, K. V. (2016). Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biologia Plantarum, 60, 201–218. https://doi.org/10.1007/s10535-016-0584-8
- Rasheed, A., Mahmood, A., Maqbool, R., Albaqami, M., Sher, A., Sattar, A., Khosa, G. B., Nawaz, M., Hassan, M. U., & Al-Yahyai, R. (2022). Key insights to develop drought-resilient soybean: A review. Journal of King Saud University – Science, 34, 102089. https://doi.org/10.1016/j.jksus.2022.102089
- Rodríguez, H., & Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances, 17, 319–339. https://doi.org/10.1016/s0734-9750(99)00014-2
- Rokhzadi, A., & Toashih, V. (2011). Nutrient uptake and yield of chickpea (Cicer arietinum L.) inoculated with plant growth promoting rhizobacteria. Australian Journal of Crop Science, 5, 44–48. https://www.scirp.org/reference/referencespapers?referenceid=3777753
- Rokhzadi, A., Asgharzadeh, A., Darvish, F., Nourmohammadi, G., & Majidi, E. (2008). Influence of plant growth-promoting rhizobacteria on dry matter accumulation and yield of chickpea (Cicer arietinum L.) under field condition. Agricultural and Food Sciences, 3, 253–257. https://www.semanticscholar.org/paper/Influence-of-plant-growth-promoting-rhizobacteria-Rokhzadi
- Rosas, S. B., André, S. J. A., Rovera, M., & Correa, N. S. (2006). Phosphate-solubilizing Pseudomonas putida can influence the rhizobia–legume symbiosis. Soil Biology and Biochemistry, 38, 3502–3505. https://doi.org/10.1016/j.soilbio.2006.05.008
- Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Wei, H. X., Paré, P. W., & Kloepper, J. W. (2003). Bacterial volatiles promote growth in Arabidopsis. Proceedings of the National Academy of Sciences, 100, 4927–4932. https://doi.org/10.1073/pnas.0730845100
- Saikia, S. P., & Jain, V. (2007). Biological nitrogen fixation with non-legumes: An achievable target or a dogma. Current Science, 92, 317–322. https://www.researchgate.net/publication/255620954_Biological_nitrogen_fixation_with_non-legumes_An_achievable_target_or_a_dogma
- Salomon, M. V., Bottini, R., De Souza Filho, G. A., Cohen, A. C., Moreno, D., Gil, M., & Piccoli, P. (2014). Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, induce abscisic acid accumulation and synthesis of defense-related terpenes in in vitro cultured grapevine. Physiologia Plantarum, 151, 359–374. https://doi.org/10.1111/ppl.12117
- Sashidhar, B., & Podile, A. R. (2010). Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. Journal of Applied Microbiology, 109(1), 1–12. https://doi.org/10.1111/j.1365-2672.2009.04654.x
- Schroth, M. N., & Hancock, J. G. (1981). Selected topics in biological control. Annual Review of Microbiology, 35, 453–476. https://doi.org/10.1146/annurev.mi.35.100181.002321
- Sgroy, V., Cassán, F., Masciarelli, O., Del Papa, M. F., Lagares, A., & Luna, V. (2009). Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Applied Microbiology and Biotechnology, 85, 371–381. https://doi.org/10.1007/s00253-009-2116-3
- Shanmugam, P. M., & Veeraputhran, R. (2000). Effect of organic manure, biofertilizers, inorganic nitrogen and zinc on growth and yield of rabi rice. Madras Agricultural Journal, 87(2), 87–90. https://doi.org/10.29321/MAJ.10.A00426
- Shehata, M. M., & El-Khawas, S. A. (2003). Effect of biofertilizers on growth parameters, yield characters, nitrogenous components, nucleic acids content, minerals, oil content, protein profiles and DNA banding pattern of sunflower (Helianthus annuus L. cv. Vedock) yield. Pakistan Journal of Biological Sciences, 6, 1257–1268. https://doi.org/10.3923/pjbs.2003.1257.1268
- Sheoran, S., Thakur, V., Narwal, S., Turan, R., Mamrutha, H. M., Singh, V., Tiwari, V., & Sharma, I. (2015). Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Applied Biochemistry and Biotechnology, 177, 1282–1298. https://doi.org/10.1007/s12010-015-1813-x
- Sohbat, Z. I. (2022). Non-photochemical quenching of chlorophyll fluorescence and its components: Recent advances. Journal of Life Science & Biomedicine, 4, 76–86. https://jlsbjournal.org/uploads/public_files/2022-07/10_chap.pdf
- Subrahmanyam, G., Kumar, A., Sandilya, S. P., Chutia, M., & Yadav, A. N. (2020). Diversity, plant growth promoting attributes, and agricultural applications of rhizospheric microbes. In Plant Microbiomes for Sustainable Agriculture (pp. 1–52). https://doi.org/10.1007/978-3-030-38453-1_1
- Sundara, B., Natarajan, V., & Hari, K. (2022). Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugarcane yields. Field Crops Research, 77, 43–49. https://doi.org/10.1016/S0378-4290(02)00048-5
- Sziderics, A. H., Rasche, F., Trognitz, F., Sessitsch, A., & Wilhelm, E. (2007). Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Canadian Journal of Microbiology, 53, 1195–1202. https://doi.org/10.1139/W07-082
- Tambekar, D. H., Gulhane, S. R., Somkuwar, D. O., Ingle, K. B., & Kanchalwar, S. P. (2009). Potential Rhizobium and phosphate solubilizers as biofertilizers from saline belt of Akola and Buldhana district, India. Research Journal of Agriculture and Biological Sciences, 5, 578–582. https://www.aensiweb.net/AENSIWEB/rjabs/rjabs/2009/578-582.pdf
- Tao, G., Tian, S., Cai, M., & Xie, G. (2008). Phosphate solubilizing and mineralizing abilities of bacteria isolated from soils. Pedosphere, 18, 515–523. https://doi.org/10.1016/S1002-0160(08)60042-9
- Turner, N. C. (2017). Turgor maintenance by osmotic adjustment, an adaptive mechanism for coping with plant water deficits. Plant, Cell & Environment, 40, 1–3. https://doi.org/10.1111/pce.12839
- Valente Lima, J., Tinôco, R. S., Olivares, F. L., Moraes, A. J. G. D., Chia, G. S., & Silva, G. B. D. (2020). Hormonal imbalance triggered by rhizobacteria enhances nutrient use efficiency and biomass in oil palm. Scientia Horticulturae, 264, 109161. https://doi.org/10.1016/j.scienta.2019.109161
- Vardharajula, S., Zulfikar Ali, S., Grover, M., Reddy, G., & Bandi, V. (2011). Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. Journal of Plant Interactions, 6, 1–14. https://doi.org/10.1080/17429145.2010.535178
- Vazquez, P., Holguin, G., Puente, M., Cortes, A. E., & Bashan, Y. (2000). Phosphate solubilizing microorganisms associated with the rhizosphere of mangroves in a semi-arid coastal lagoon. Biology and Fertility of Soils, 30, 460–468. https://doi.org/10.1007/s003740050024
- Whitelaw, M. A. (2000). Growth promotion of plants inoculated with phosphate solubilizing fungi. Advances in Agronomy, 69, 99–151. https://doi.org/10.1016/S0065-2113(08)60948-7
- Xiao, C. Q., Chi, R. A., Huang, X. H., & Zhang, W. X. (2008). Optimization for rock phosphate solubilization by phosphate-solubilizing fungi isolated from phosphate mines. Ecological Engineering, 33, 187–193. https://doi.org/10.1016/j.ecoleng.2008.04.001
- Yahya, A., & Azawi, S. K. A. (1998). Occurrence of phosphate solubilizing bacteria in some Iranian soils. Plant and Soil, 117, 135–141. https://doi.org/10.1007/BF02206266
- Yasmin, H., Bano, A., Wilson, N. L., Nosheen, A., Naz, R., Hassan, M. N., Ilyas, N., Saleem, M. H., Noureldeen, A., & Ahmad, P. (2022). Drought-tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiologia Plantarum, 174, e13497. https://doi.org/10.1111/ppl.13497
- Zaddy, E., & Perevolosky, A. (1995). Enhancement of growth and establishment of oak seedlings by inoculation with Azospirillum brasilense. Forest Ecology and Management, 72, 81–83. https://www.academia.edu/33819891/
- Zaddy, E., Perevolosky, A., & Okon, Y. (1993). Promotion of plant growth by inoculation with aggregated and single-cell suspension of Azospirillum brasilense. Soil Biology and Biochemistry, 25, 819–823. https://doi.org/10.1007/s00248-004-0148-x
- Zhang, W., Xie, Z., Zhang, X., Lang, D., & Zhang, X. (2019). Growth-promoting bacteria alleviates drought stress of Glycyrrhiza uralensis through improving photosynthesis characteristics and water status. Journal of Plant Interactions, 14, 580–589. https://doi.org/10.1080/17429145.2019.1680752






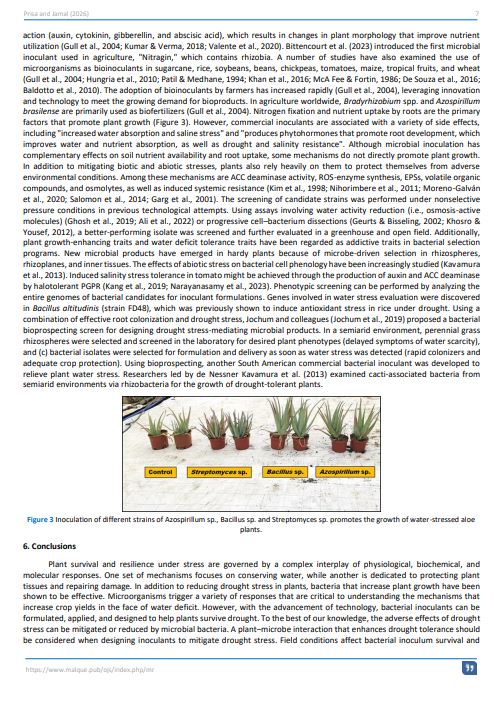


Condividi:
- Invia un link a un amico via e-mail (Si apre in una nuova finestra) E-mail
- Condividi su X (Si apre in una nuova finestra) X
- Stampa (Si apre in una nuova finestra) Stampa
- Condividi su WhatsApp (Si apre in una nuova finestra) WhatsApp
- Condividi su Facebook (Si apre in una nuova finestra) Facebook
- Condividi su Telegram (Si apre in una nuova finestra) Telegram
- Condividi su X (Si apre in una nuova finestra) X
- Condividi su Threads (Si apre in una nuova finestra) Threads
- Condividi su LinkedIn (Si apre in una nuova finestra) LinkedIn

